We are a certified manufacturer of medical cables, sensors and accessories. also offer OEM/ODM medical cable solution for your own-brand device.
Timely response to follow up on customer requests, and accurately analyze customer needs, strive to propose preliminary solutions within 3 hours;




Within 24 hours, we organize the business department and R&D department to discuss the feasibility of the program in detail and determine the project establishment;




Match the requirements with the R&D technical department within 3 days, produce the drawing plan and finalize the final feasible plan with the client;

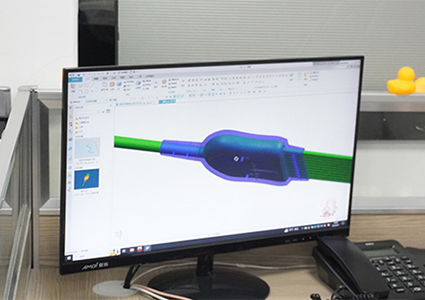
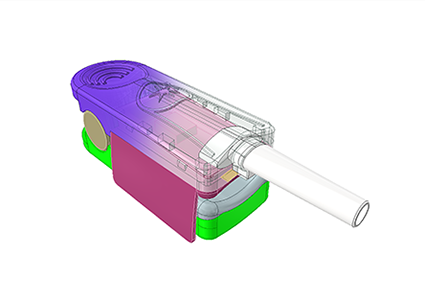
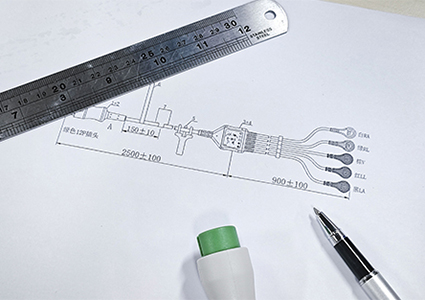
Rapidly prepare materials based on confirmed feasible solutions and procure raw materials in preparation for the next step of mold development;




Determine the mold design and quote the entire project;




Finish making and adjusting the molds within 7 days to ensure the smooth production of samples;

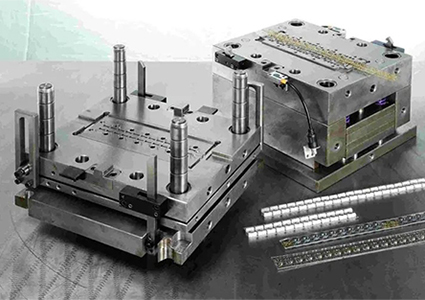

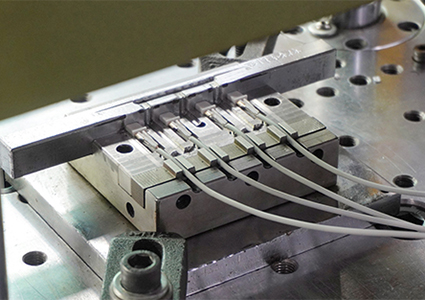
Sample production within 15 days;




Completion of technical and parametric validation tests based on samples;
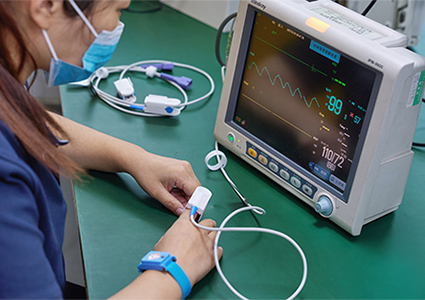
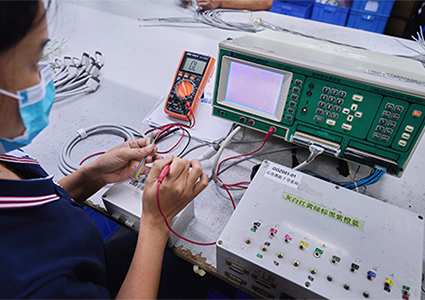


Mass production within 20 days with guaranteed production yields;



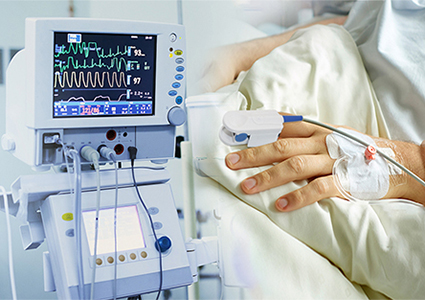
With over decades experience in this field allows us to meet custom requirements for cable easier.we operates our manufacturing plants under a quality management system based on the requirements of EN ISO13485:2012 and in compliance with the requirements of Regulation (EU) 2017/745.